Abstract
Background: Therapeutic options remain limited for elderly acute myeloid leukemia (AML) considering the poor tolerance to intensive chemotherapy. Venetoclax in combination with hypomethylation agents (HMAs) or low-dose cytarabine (LDAC) has been shown achieved significantly improved efficacy in elderly AML patients. Recently, we reported that an intensive chemotherapy regimen of venetoclax plus daunorubicin and cytarabine (DAV regimen) showed high complete remission (CR) rates of 91% after one cycle of induction therapy in newly diagnosed adult young AML patients, indicating the strong potential of the venetoclax-based intensive therapy to induce rapid remission (Lancet Hematol, 2022). For fit elderly AML patients, a prospective, phase Ib study demonstrated that venetoclax plus "2 + 5" (idarubicin and cytarabine) regimen achieved high CR rates of 68% (CR and CR with incomplete count recovery [CRi] rate 97%) in fit elderly patients with de novo AML. Unfortunately, delayed hematologic recovery, especially prolonged thrombocytopenia, was observed during consolidation therapy. Thus, an optimized venetoclax-based modified intensive therapy with the hope to achieve higher CR rates and better tolerance and improve long-term survival are urgently needed.
Objective: To evaluate the efficacy and safety of modified intensive induction of venetoclax in combination with "2 + 5" (daunorubicin and cytarabine) chemotherapy and the following intermediate-dose cytarabine plus venetoclax consolidation in untreated fit de novo elderly AML.
Design, setting and participants: Retrospective clinical trial conducted in the First Affiliated Hospital, Zhejiang University College of Medicine, China. Eligible patients (≥60 years old) with de novo AML (exclude acute promyelocytic leukemia) were enrolled since March 9, 2021, with final follow-up in July 15,2022.
Interventions: Patients were treated with induction chemotherapy included daunorubicin (60mg/m2 days 1-2, intravenously), cytarabine (100mg/m2 days 1-5, intravenously), and venetoclax (100mg day 3, 200mg day 4, 400mg days 5-10, orally), followed by 3 consolidation cycles of venetoclax (400mg days 1-14, orally) in combination with intermediate-dose cytarabine (1g/m2 days 1-3, intravenously) and post-remission bone marrow transplantation when applicable. Venetoclax was administered for the maintenance therapy for one year (200mg days 1-14 in each 28-day cycle, orally).
Main outcomes and measures: The primary endpoints included the rate of composite complete remission (CRc) after one cycle of induction treatment, including CR/CRi. The secondary endpoints were measurable residual disease detected by muti-parameter flow cytometry (MFC-MRD), adverse events, event-free survival (EFS) and overall survival (OS).
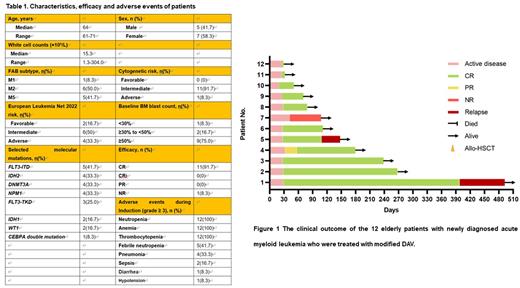
Results: Twelve patients were enrolled. Median age was 64 years old (range, 61-71), with poor-risk in 33.3% (4/12) of patients (European LeukemiaNet 2022 risk). Other characteristics of patients were listed in Table 1. The CR rate were 91.7% (11/12) (Table 1). All patients with poor-risk achieved CR. Measurable residual disease-negative composite CR was attained in 100.0% (11 out 11) of total patients achieved CR (Table 1, Figure 1). Common adverse events (>30%) included thrombocytopenia. neutropenia, anemia, febrile neutropenia, nausea, and pneumonia. The main grade ≥ 3 hematologic toxicities during induction were neutropenia (100%), anemia (100%) and thrombocytopenia (100%). The main grade ≥ 3 nonhematologic toxicities during induction were febrile neutropenia (41.7%) and pneumonia (33.3%) (Table 1). No tumor lysis syndrome was observed. The median follow-up was 108 days. The median EFS was 398 days, with an estimated 1-year EFS rate of 75.0%. The median OS was not achieved, with an estimated 1-year OS rate of 100% (Table 1). 9 out of 11 patients achieving CR maintained the MRD-negative status until the last follow-up (Figure 1). No deaths occurred among the patients during follow-up.
Conclusions: The novel combination of "2+5" (daunorubicin and cytarabine) with venetoclax (modified DAV regimen) showed encouraging efficacy and good tolerability in untreated fit elderly patients with de novo AML, with high CR rate and deep remission.
Figure legends Figure 1 The clinical outcome of the 12 elderly patients with newly diagnosed acute myeloid leukemia who were treated with modified DAV.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal